24
2014-11
BrosMed Announces the Launch of a New Company Logo and Website
November 24, 2014, we are proud to announce the launch of a new company logo and release our new website, designed with a fresh new look and user-friendly navigation, updated with the latest information about our products and services, as part of the ongoing evolution of our company’s brand and Visual Identity (VI).
2014-11-24
08
2014-10
October 8, 2014— Following the FDA approval of Artimes™ Balloon Dilatation Catheter in September, BrosMed announced FDA 510(k) clearance for Apollo™ Balloon Dilatation Catheter which is now approved and ready for sale in the United States of America and the North America market for the interventional cardiovascular use.
2014-10-08
17
2014-09
September 17, 2014— Following the CE approval and launch Artimes™ Balloon Dilatation Catheter, BrosMed announced FDA 510(k) clearance for Artimes™ Balloon Dilatation Catheter which is now approved and ready for sale in the United States of America and the North America market for the interventional cardiovascular use.
2014-09-17
15
2014-09
BrosMed Medical is going to exhibit Artimes™ & Artimes™ CTO semi-compliance, Apollo™ Non-compliance high pressure PTCA series, Minerva™ PTA, Freepath™ CSI, Conger™ Hydrophilic Wire and ONE-STOP full range accessories at 25th Great Wall International Congress of Cardiovascular (GWICC) at China National Convention Center, Beijing China, during October 16-19th 2014.
2014-09-15
02
2014-09
BrosMed Establishes Europe Subsidiary in the Netherlands
September 2, 2014, BrosMed Medical has announced the establishment of a subsidiary company in the Netherlands, BrosMed Medical B.V.
2014-09-02
31
2014-07
BrosMed Medical is going to exhibit our outstanding PTCA balloon catheter series and full range of Accessories for PCI at FIME show in Miami Beach Convention Center, Florida USA, during August 6-8th 2014.
2014-07-31
10
2014-07
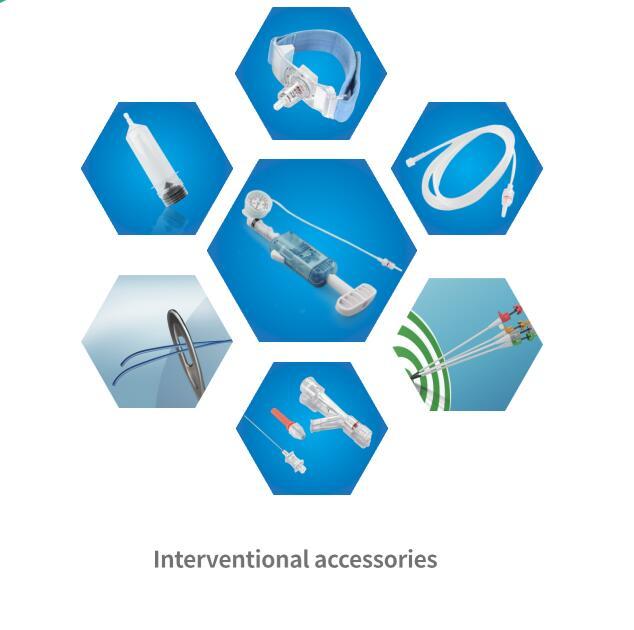
July, 10, 2014— Following the CE approval and launch Artimes™ and Apollo™ Balloon Dilatation Catheter, BrosMed announced CE Mark and Launch of Fourteen (14) types of interventional accessories which are now approved in EU for the cardiology and radiology procedures.
2014-07-10
25
2014-02
BrosMed Announces CE Mark and Launch for Apollo™ Balloon Dilatation Catheter
February 25, 2014— Following the CE approval and launch Artimes™ Balloon Dilatation Catheter, BrosMed announced CE Mark and Launch of its Apollo™ Balloon Dilatation Catheter which is now approved in the EU for the interventional cardiovascular use.
2014-02-25