BrosMed and Cordis Partner to Promote the Next-Generation Drug-Coated Balloon VaSecure™ in China Market
Release time:
2025-02-12
On February 10, BrosMed and Cordis signed an agreement for the exclusive distribution of VaSecure™, BrosMed’s next-generation drug-coated peripheral balloon catheter in the China market.
On February 10, BrosMed and Cordis signed an agreement for the exclusive distribution of VaSecure™, BrosMed’s next-generation drug-coated peripheral balloon catheter in the China market. Featuring globally pioneering technology, VaSecure™ successfully achieves "zero drug loss" and ensures "long-term retention," addressing the three major challenges of “drug loss, drug transfer, and drug retention” that have long affected peripheral drug-coated balloons. This breakthrough is set to usher in a new era for the advancement of peripheral drug-coated balloon technology.
As one of the global leaders in cardiac and peripheral vascular intervention, Cordis will accelerate the commercialization of this product following the signing of the exclusive distribution agreement with BrosMed for the China market. This partnership will also help Cordis further expand its peripheral interventional product line, offering a wide range of localized products to medical professionals and patients in China.

Innovative Design
“Zero Drug Loss” is going to be EPIC
Peripheral DCB has been widely used in the treatment of peripheral arterial disease. Now, it has once again made breakthroughs in innovation.
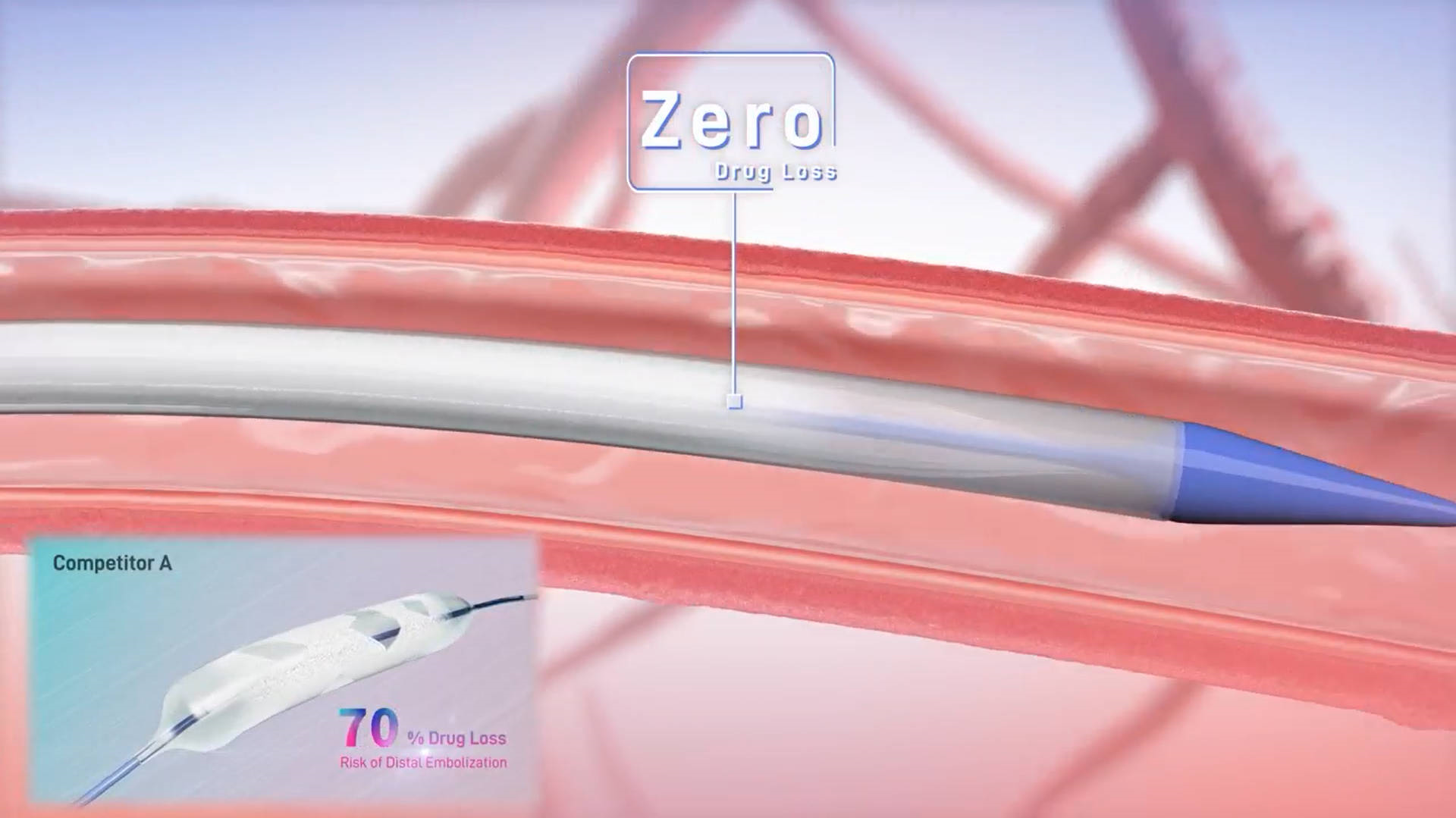
After several years of dedicated research and development, BrosMed has launched the pioneer “VaSecure™ Paclitaxel-coated PTA Balloon Catheter”, the world's first drug-coated balloon catheter featuring a protective sheath throughout the process from entering the sheath to reaching the target lesion, as well as adopting the patented “Micro-needle-crystals” coating technology, successfully solving the three major problems of current peripheral DCBs: “Drug loss, drug transfer, and drug retention”. It provides a brand-new solution for the treatment of peripheral diseases.
It is known that the main challenges with current peripheral DCBs in clinical application are severe drug loss, low transfer efficiency, and short retention time. Due to the structural characteristics of DCBs, both hydrophilic and lipophilic excipients face challenges in preventing drug loss during balloon delivery and the subsequent washing effect from blood flow. Drug loss not only reduces the effective drug transfer to the vessel wall but also increases the risk of distal embolization. In addition, the low drug transfer rate and the insufficient retention time of the drug at the target lesion also contribute to the instability of clinical efficacy. These factors have limited the effectiveness of peripheral DCB in broader clinical applications.
VaSecure™ Paclitaxel-coated PTA Balloon Catheter features an innovative delivery platform with protective sheath covered throughout the process from entering the sheath to reaching the target lesion, which ensures zero drug loss during the delivery process, reduces the risk of distal embolization, and enhances the efficiency of drug transfer to the vessel wall.
In addition, it applies a novel “Micro-needle-crystals” drug coating and patented spraying technology, with penetrated drug delivery mode, which greatly improves drug transfer efficiency, and ensures long drug retention time in blood vessel wall.
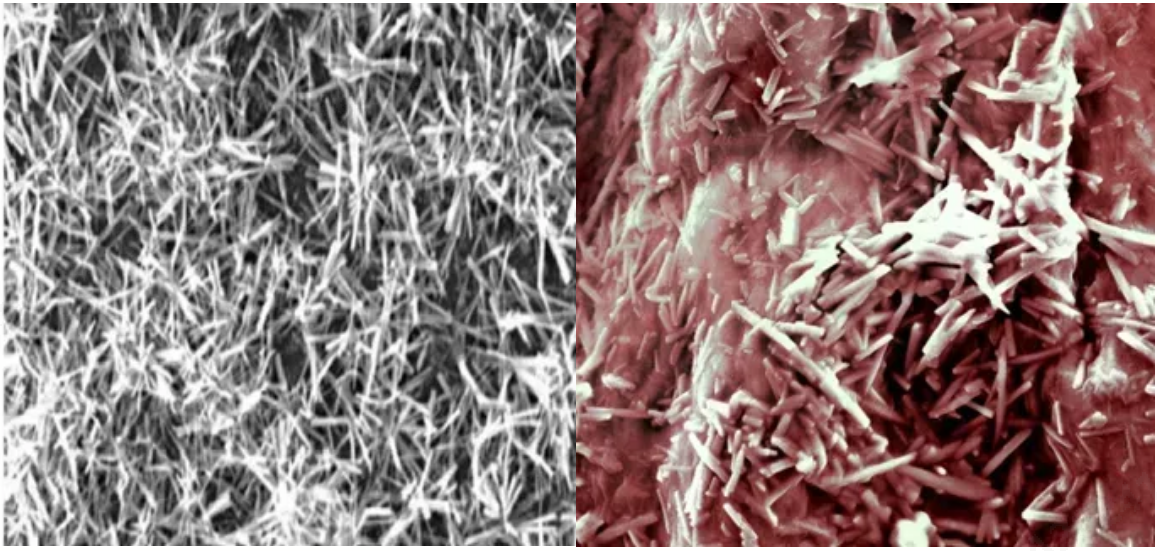
| Left: | Right: “Penetrated” drug delivery mode |
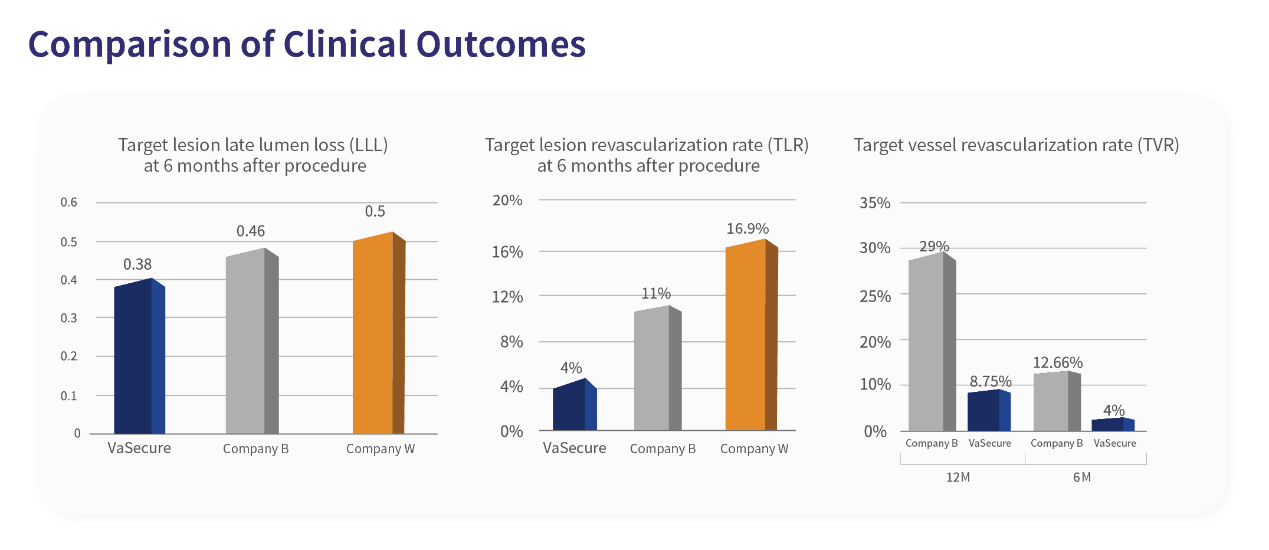
Superior product design leads to excellent clinical outcomes. The clinical trial data of VaSecure™ are convincing. The late lumen loss (LLL) of the target lesion was only 0.38mm at 6 months postoperatively and the target lesion revascularization rate (TLR) was only 4% at 6 months postoperatively.
VaSecure™ is currently approved by the NMPA and is indicated for percutaneous transluminal angioplasty in patients with stenotic or occlusive lesions of the superficial femoral artery (SFA) and proximal popliteal artery (PPA). Additionally, VaSecure™ is under CE registration and is poised to enter the international market, expanding its reach globally.
Complementary Strengths, Win-Win Cooperation
BrosMed and Cordis, longstanding partners, are once again collaborating to drive the commercialization of the VaSecure™ drug-coated peripheral balloon catheter.
With the signing of the exclusive distribution agreement, Cordis will leverage its professional and well-established marketing network to help BrosMed swiftly introduce the innovative VaSecure™ Paclitaxel-coated PTA Balloon Catheter to the China market.
At the same time, this agreement will further expand Cordis's peripheral interventional product line, filling the gap in its product offering for drug-eluting treatments in peripheral interventions, and creating new avenues for business growth.
In the future, BrosMed will continue to embrace open cooperation, combining global expertise with Chinese innovation, and collaborate with partners to meet unmet clinical needs, contributing to the advancement of vascular intervention around the world.
Superior product design leads to excellent clinical outcomes. The clinical trial data of VaSecure™ are convincing. The late lumen loss (LLL) of the target lesion was only 0.38mm at 6 months postoperatively and the target lesion revascularization rate (TLR) was only 4% at 6 months postoperatively.
Join with Us!
Please fill out this form and we will get in touch within 24 hours.
* Note: Please be sure to fill in the information accurately and keep the communication unblocked. We will get in touch with you as soon as possible.
More news
Save the Date December 10–11, 2025 and visit us at GulfPCR 2025 to explore more highlights!
2025-12-03
Webinar Invitation | Mastering PTA: From Peripheral Arteries to AV Fistulas II
Join us at 08:30–10:00 (UTC+1) on Thursday, November 27, 2025!
2025-11-19
Webinar Invitation | Innovations and Novel Strategies in CHIP PCIⅡ
Mark your calendar: Wednesday, October 22 🕒 15:00-17:30 (Beijing Time) | 09:00-11:30 (CEST)
2025-10-14
Save the Date October 3-4, 2025 and visit us at EBC 2025 to explore more highlights!
2025-09-19