BrosMed’s POT™ NC PTCA Balloon Dilatation Catheter Receives FDA 510(k) Clearance
Release time:
2023-04-12
April 12, 2023—BrosMed Medical has received FDA 510(k) clearance for the POT™ NC PTCA Balloon Dilatation Catheter, which is the world’s first dedicated balloon for proximal optimization technique (POT) and distal optimization technique (DOT) in bifurcation stenting. With this certification, a specialized tool in the treatment of precise PCI for bifurcation lesions is available to US physicians.
April 12, 2023—BrosMed Medical has received FDA 510(k) clearance for the POT™ NC PTCA Balloon Dilatation Catheter, which is the world’s first dedicated balloon for proximal optimization technique (POT) and distal optimization technique (DOT) in bifurcation stenting. With this certification, a specialized tool in the treatment of precise PCI for bifurcation lesions is available to US physicians.
Coronary bifurcation accounts for 15-20% of all percutaneous coronary interventions (PCI), which is still expected to be one of the most challenging lesions in interventional cardiology in terms of a low procedural success rate as well as long-term cardiac events.

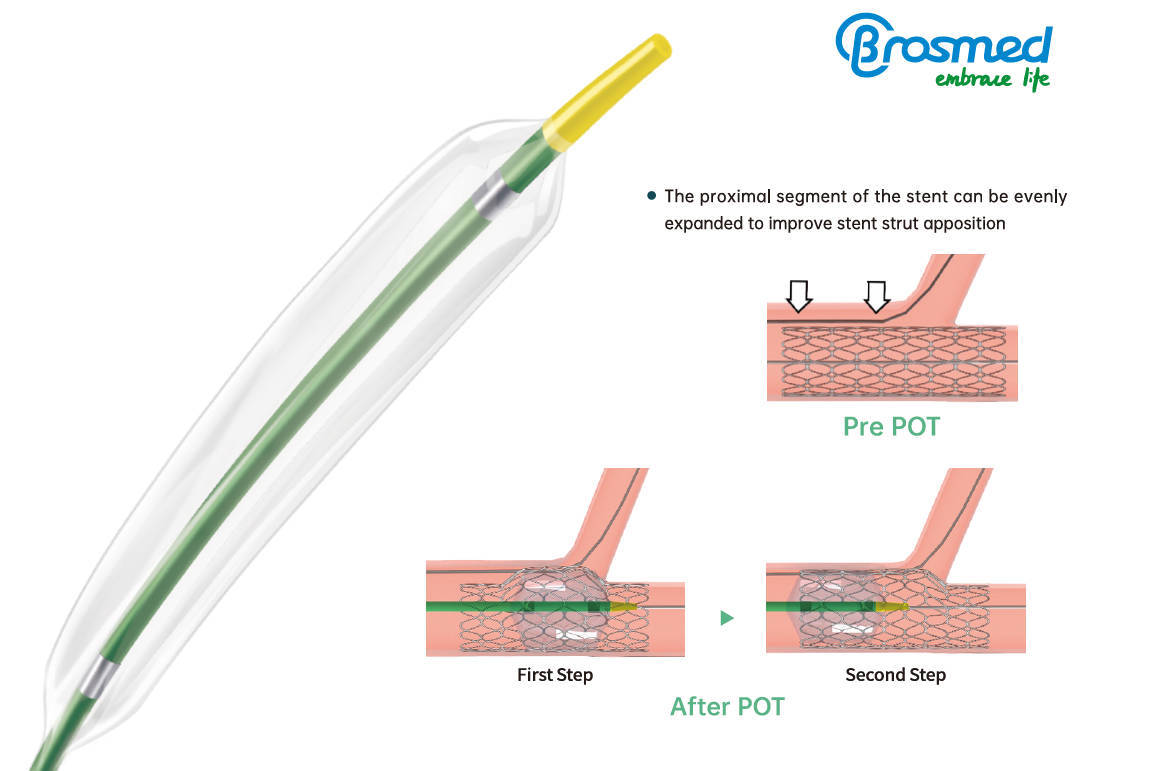
The proximal optimization technique (POT) has been proposed as an effective strategy to achieve optimal stent expansion and apposition in the proximal segment in coronary bifurcation. It is a straightforward technique whereby a short, appropriately-sized balloon like POT™ is inflated in the main vessel just proximal to the carina. The advantages of this technique include reducing the risk of side branch compromise related to shifting of the carina, improving stent apposition in the proximal main vessel, as well as facilitating side branch access after main vessel stent implantation.
As the special-designed balloon for POT and DOT technique, POT™ reigns as the best choice of treating bifurcations undoubtedly. The POT™ PTCA catheter is a non-compliant balloon designed with extra short balloon shoulders and precise apposition performance in order to reduce longitudinal balloon growth and minimize the potential for vessel trauma outside the treatment area in bifurcation stenting.
Prof. Shao-Liang Chen, the inventor of DK-crush, “A real POT™ balloon innovated by BrosMed has provided the final solution for either POT or DOT. POT™ PTCA balloon catheter offers tremendous promise and adds a state-of-the-art coronary balloon to BrosMed leading products portfolio.”
BrosMed will strive to continue developing innovative devices to address the most complicated clinical challenges and make contributions to physicians and patients worldwide, as well as the development of the medical industry.
For more information about POT™ NC PTCA, please visit BrosMed website or contact us via e-mail: Sales@brosmed.com.
Join with Us!
Please fill out this form and we will get in touch within 24 hours.
* Note: Please be sure to fill in the information accurately and keep the communication unblocked. We will get in touch with you as soon as possible.
More news
Webinar Invitation | Innovations and Novel Strategies in CHIP PCI
Mark your calendar: Wednesday, March 26 🕒 18:00-19:30 (Beijing Time) | 11:00-12:30 (CET)
2025-03-19
On February 10, BrosMed and Cordis signed an agreement for the exclusive distribution of VaSecure™, BrosMed’s next-generation drug-coated peripheral balloon catheter in the China market.
2025-02-12
BrosMed Medical Obtains CE Mark for RevoEdge™ High-Pressure Cutting Balloon
BrosMed Medical is excited to announce that it has obtained CE mark for the RevoEdge High-Pressure Coronary Cutting Balloon under the European Medical Device Regulation (MDR). Redefining the standards of cutting balloons, this cutting-edge device is designed to offer unparalleled cutting performance and crossability.
2025-01-07
Meet BrosMed at ESC Congress 2024
BrosMed Medical is delighted to participate in the ESC Congress 2024 from August 30th to September 2nd in London, UK.
2024-08-15
BrosMed Medical Announces Scott J. Addonizio as Chief Operating Officer
2024 August 1st – BrosMed Medical is pleased to announce the appointment of Scott J. Addonizio as its Chief Operating Officer (COO), effective immediately. Mr. Scott J. Addonizio brings a wealth of experience and expertise to BrosMed, with a distinguished career spanning almost 30 years in the medical device industry. Prior to joining BrosMed, Mr. Addonizio served as SVP at Cordis and COO at MedAlliance Swiss Medical Technology.
2024-08-01
CTO&CHIP PCI – Insights from the clinical practice (Ⅱ)
July 30, 2024 @16:00-17:30 (Beijing Time) Join BrosMed Medical on Tuesday, July 30 for an enlightening discussion that unites cardiologists from Malaysia, China, India, Tunisia and Portugal, where clinical expertise harmoniously fuses with CTO&CHIP innovation. At this exclusive webinar, we’re orchestrating a confluence of valuable insights, drawing from the collective wisdom of seasoned interventional cardiologists.
2024-07-26