BrosMed Medical Earns MDSAP Certification
Release time:
2023-06-21
Dongguan-June 21, 2023-Today BrosMed announced that it has earned the Medical Device Single Audit Program (MDSAP) certification, indicating that the single regulatory audit to BrosMed satisfies the requirements of multiple regulatory jurisdictions participating in the program.
Dongguan-June 21, 2023-Today BrosMed announced that it has earned the Medical Device Single Audit Program (MDSAP) certification, indicating that the single regulatory audit to BrosMed satisfies the requirements of multiple regulatory jurisdictions participating in the program.

The MDSAP is a unique program that brings the internationally leading regulatory agencies together to perform a single regulatory audit of a medical device manufacturer´s quality management system to satisfy the requirements of multiple regulatory jurisdictions, including the Therapeutic Goods Administration of Australia, Brazil’s Agência Nacional de Vigilância Sanitária, Health Canada, Japan’s Ministry of Health, Labour and Welfare and Japanese Pharmaceuticals and Medical Devices Agency, and the U.S. Food and Drug Administration. This certification confirms BrosMed’s compliance with the high standards and regulatory requirements of most leading countries in the world.
“This certification is a key achievement for us on the road to global commercialization. It is a critical step forward in offering our intravascular interventional solutions to additional markets and reinforces our commitment to quality and safety,” said Winnie Huang, BrosMed Quality Director.
At present, BrosMed has more than 20 product series sold to over 80 countries and regions. BrosMed will continue to reinforce our commitment to high-standard quality and safety and provide more innovative products for patients around the globe.
Join with Us!
Please fill out this form and we will get in touch within 24 hours.
* Note: Please be sure to fill in the information accurately and keep the communication unblocked. We will get in touch with you as soon as possible.
More news
BrosMed Medical is delighted to participate in the ASVS 2025 Congress from August 29th to 31st in Singapore.
2025-07-30
Save the Date August 6–8, 2025 and visit us at Booth #9 to explore more highlights!
2025-07-30
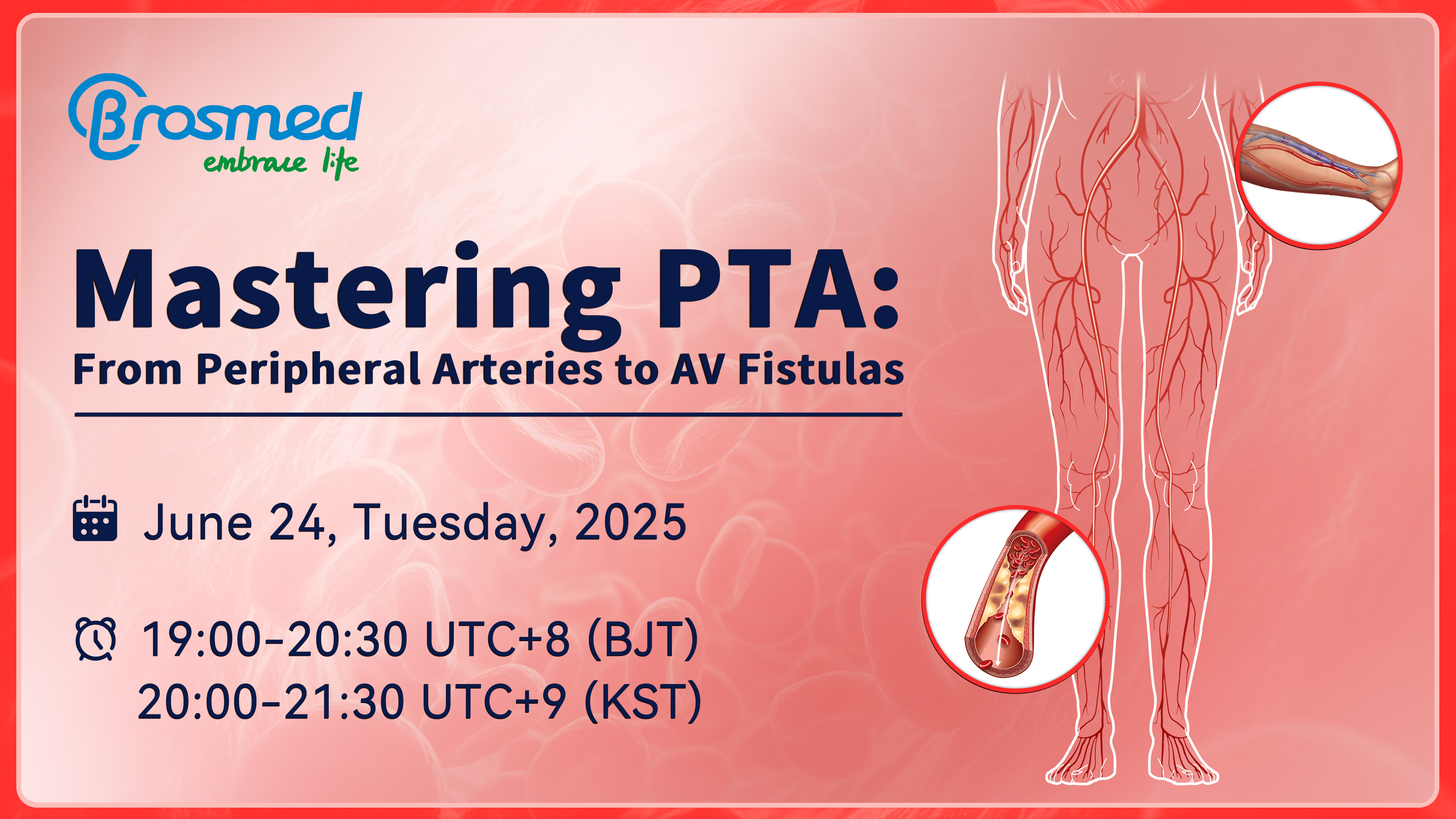
Webinar Invitation | Mastering PTA: From Peripheral Arteries to AV Fistulas
Mark your calendar: Tuesday, June 24 🕒 19:00-20:30 UTC+8 (BJT) | 20:00-21:30 UTC+9 (KST)
2025-06-18
Join Us at AngioPicture 2025 for the AVF Seminar!
BrosMed Medical cordially invites you to attend our AVF Seminar at AngioPicture 2025, held on May 30th at Pullman Sochi Centre, Sochi, Russia.
2025-05-20
BrosMed Announced CE MDR Approval of New Generation Conger™ Plus Hydrophilic Guidewires
Designed for superior crossability and maneuverability in peripheral interventions, Conger Plus now offers 116 models with expanded sizes (OD 0.018'' to 0.038'') and lengths (up to 300cm).
2025-04-28