ESVS 2023 丨Prof. Qizhuang Jin’s team: 12-month results of a peripheral intervention scoring balloon dilatation catheter (BrosMed Tri-Wedge™) in the treatment of stenotic lesions of arteriovenous fistula
Release time:
2023-10-19
The 37th Annual Meeting of the European Society for Vascular Surgery 2023 (ESVS 2023) was successfully held in Belfast, United Kingdom, from September 26 to 29. The ESVS Annual Meeting is globally recognized as one of the most important congresses on vascular surgery, attracting many specialists, professors, and physicians from all over the world every year. During the 4-days event, a wide range of educational webinars, interactive case discussions and hands-on workshops were organized to provide participants with a wide range of advanced training opportunities from the 5 academy areas: aortic, carotid, PAD, vascular access and venous.
The 37th Annual Meeting of the European Society for Vascular Surgery 2023 (ESVS 2023) was successfully held in Belfast, United Kingdom, from September 26 to 29. The ESVS Annual Meeting is globally recognized as one of the most important congresses on vascular surgery, attracting many specialists, professors, and physicians from all over the world every year. During the 4-days event, a wide range of educational webinars, interactive case discussions and hands-on workshops were organized to provide participants with a wide range of advanced training opportunities from the 5 academy areas: aortic, carotid, PAD, vascular access and venous.
During the Annual Meeting, Prof. Qizhuang Jin’s team from Peking University First Hospital delivered a wonderful keynote speech on “Scoring balloon angioplasty for dysfunctional arteriovenous fistulas-A multicenter randomized controlled trial”. The presentation shares the results of a 12-month study of a peripheral intervention scoring balloon (BrosMed Tri-Wedge™) in the treatment of stenotic lesions of autologous arteriovenous fistulas.

Background of the study
Vascular Stenosis is one of the common complications of arteriovenous fistula (AVF) and is the main cause of AVF thrombosis and eventual loss of function. Percutaneous transluminal angioplasty (PTA) is the preferred choice of AVF stenosis treatment. However, the stenosis of hemodialysis access is more manifested as intimal hyperplasia (IH) high resistance lesions, the stenosis is easy to recur with poor prognosis after traditional PTA with ordinary PTA balloon dilatation catheter. The application of special balloon can increase the success rate. Nowadays the high-pressure balloon will cause excessive damage to the blood vessel. Also, there is a risk of blood vessel rupture if improper operation of the scoring balloon. How to safely and effectively treat the stenotic lesions of AVF has become a major challenge in clinical treatment.
Purpose of the study
Few clinical studies have been reported on the treatment of stenotic lesions of autologous arteriovenous fistulae with scoring balloon, and the aim of this study was to clarify the safety and efficacy of a peripheral intervention scoring balloon dilatation catheter in the treatment of stenotic lesions of autologous arteriovenous fistulae.
Study Design
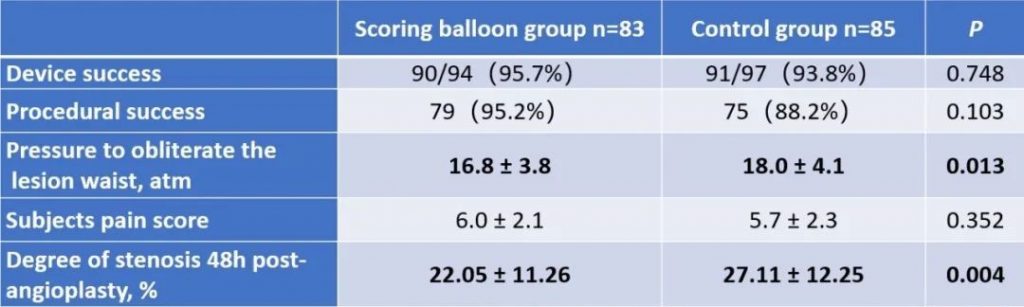
This multicenter, randomized, controlled, non-inferiority clinical trial was led and completed by Prof. Qizhuang Jin, the Chief Physician of Nephrology at Peking University First Hospital. There are 168 qualified subjects from 6 study centers in China enrolled in this study. Subjects were randomly assigned to the experimental group (BrosMed Tri-Wedge™ PTA Scoring Balloon Dilatation Catheter, n=83) and the control group (High Pressure PTA Balloon Catheter, n=85). The primary endpoint is technical success rate, defined as the target lesion residual stenosis<30% immediately after angioplasty. The secondary endpoints are device success, procedural success, dilating pressure, subject pain score, 48 hours stenosis rate after angioplasty, and target lesion primary patency (TLPP) at 12 months. Safety endpoints include vascular rupture, serious adverse events, death, heart failure, stroke, and device defect.
Study Results
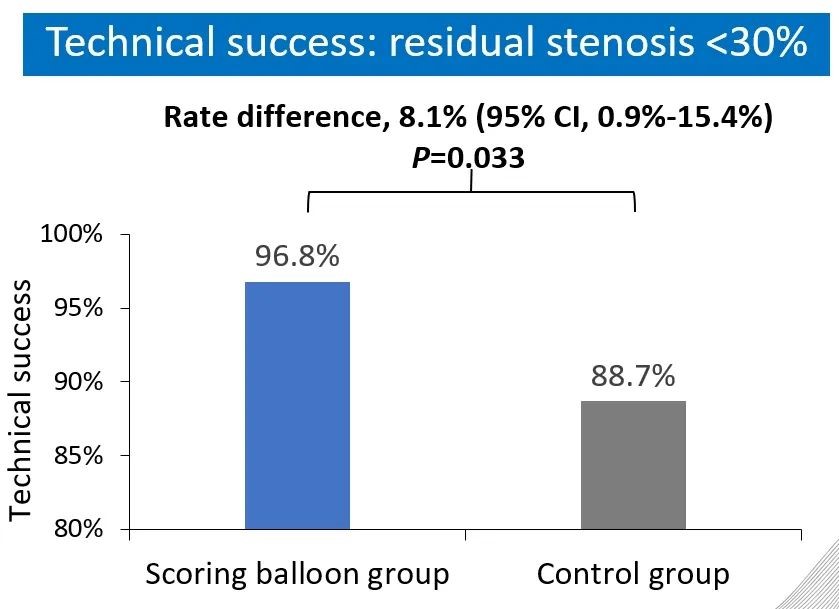
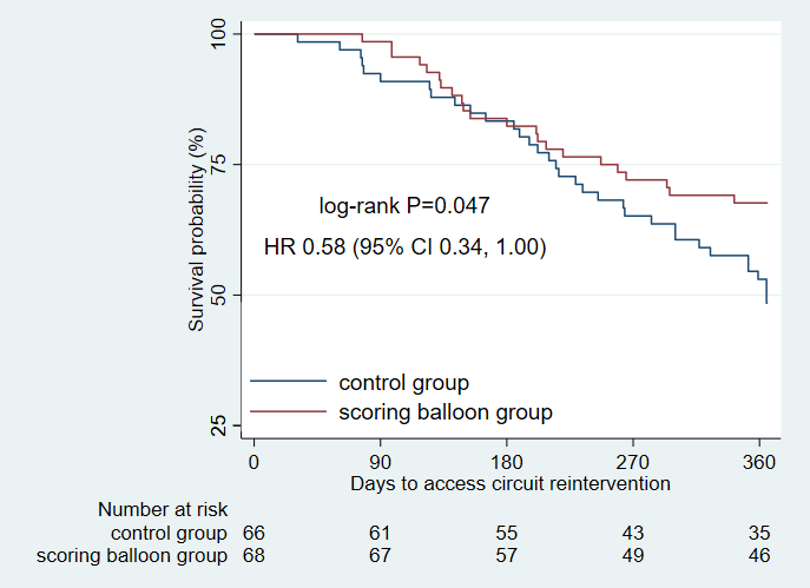
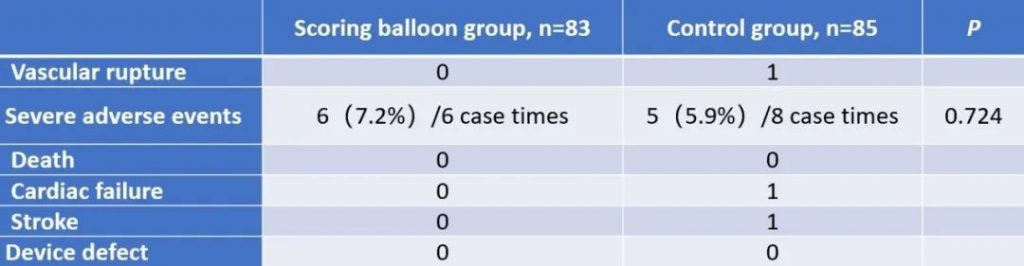
The results show that the technical success rate of experimental group (BrosMed Tri-Wedge™ PTA Scoring Balloon Dilatation Catheter) was 96.8%, while the control group only achieved 88.7% (P=0.033). At 12 months after the procedure, target lesion primary patency for BrosMed Tri-Wedge™ PTA Scoring Balloon group was 67.6%, and that of the control group was 48.5% (P=0.047). The average dilating pressure of stenosis lesions in the two groups was 16.8±3.8 atm and 18.0±4.1 atm (P=0.013), and 48 hours degree of stenosis after angioplasty was 22.05±11.26% and 27.11±12.25% (P=0.004) respectively. The incidence rates of serious adverse events in the two groups were 6 (7.2%)/6 case times and 5 (5.9%)/8 case times (P=0.724) respectively. There was no vascular rupture, death, heart failure, or stroke events occurring in BrosMed Tri-Wedge™ PTA Scoring Balloon group. However, there were 1 case of vascular rupture, 1 case of heart failure, 1 case of stroke, and no death happening in the control group. There were no events related to device defects in either group.
Overview
Technical success rates for BrosMed Tri-Wedge™ PTA Scoring Balloon and normal high-pressure balloon were similar, with no greater adverse events. BrosMed Tri-Wedge™ PTA Scoring Balloon was able to achieve adequate expansion under lower dilation pressures and result in better lumen maintenance 48 hours after the procedure. Additional data are needed in the future to evaluate the long-term efficacy of BrosMed Tri-Wedge™ PTA Scoring Balloon angioplasty for arteriovenous fistula stenosis.
Experts Profile

Prof. Qizhuang Jin
- Chief Physician of Nephrology in Peking University First Hospital, China
- Member of Asian Pacific Society of Dialysis Access (APSDA)
- Primary investigator of BrosMed Tri-Wedge™ PTA Scoring Balloon Dilatation Catheter Trial in China

Yin Yanqi M.D.
- Attending Doctor of Nephrology in Peking University First Hospital, China
- Members of Asia-Pacific Angiology Academic Association
- Sub-investigator of BrosMed Tri-Wedge™ PTA Scoring Balloon Dilatation Catheter Trial in China
Join with Us!
Please fill out this form and we will get in touch within 24 hours.
* Note: Please be sure to fill in the information accurately and keep the communication unblocked. We will get in touch with you as soon as possible.
More news
BrosMed Medical is delighted to participate in the ASVS 2025 Congress from August 29th to 31st in Singapore.
2025-07-30
Save the Date August 6–8, 2025 and visit us at Booth #9 to explore more highlights!
2025-07-30
Webinar Invitation | Mastering PTA: From Peripheral Arteries to AV Fistulas
Mark your calendar: Tuesday, June 24 🕒 19:00-20:30 UTC+8 (BJT) | 20:00-21:30 UTC+9 (KST)
2025-06-18
Join Us at AngioPicture 2025 for the AVF Seminar!
BrosMed Medical cordially invites you to attend our AVF Seminar at AngioPicture 2025, held on May 30th at Pullman Sochi Centre, Sochi, Russia.
2025-05-20
BrosMed Announced CE MDR Approval of New Generation Conger™ Plus Hydrophilic Guidewires
Designed for superior crossability and maneuverability in peripheral interventions, Conger Plus now offers 116 models with expanded sizes (OD 0.018'' to 0.038'') and lengths (up to 300cm).
2025-04-28